I have been keeping an eye out for suitable materials to make a Zamboni battery. The battery above uses small strips from discarded medical ECG electrodes to generate 2.9V.
“Continue reading” for more details and photos.
A Zamboni battery or Duluc dry pile is a battery made of thin discs of metal (often copper-zinc) separated by paper which is kept slightly conductive by the humidity. By stacking up thousands of these a battery of thousands of volts at an extremely low current can be made. This is enough to drive devices that run on charge such as Franklin bells. The Oxford Electric bell has been running since 1840.
I have used electrodes that are used for taking ECG’s (electrocardiograms) of which 10 are used for each recording and are single use then discarded. The electrodes are silver coated plastic coated with a silver chloride impregnated gel which is sticky. The silver is exposed on one corner to allow the electrical connection to a crocodile clip.
So could these be used to make multiple cells with stacking of hundreds of these pads as electrodes?
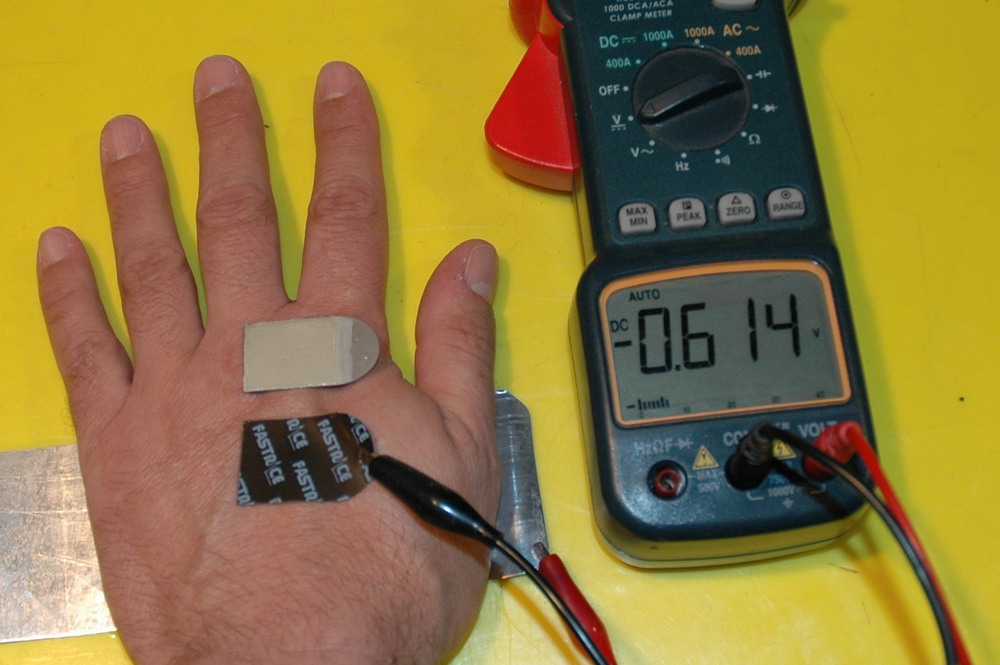 The electrode on my hand and the underside of another one. I have also connected it to a meter to show that 0.6 V is generated across this cell to my hand.
The electrode on my hand and the underside of another one. I have also connected it to a meter to show that 0.6 V is generated across this cell to my hand.
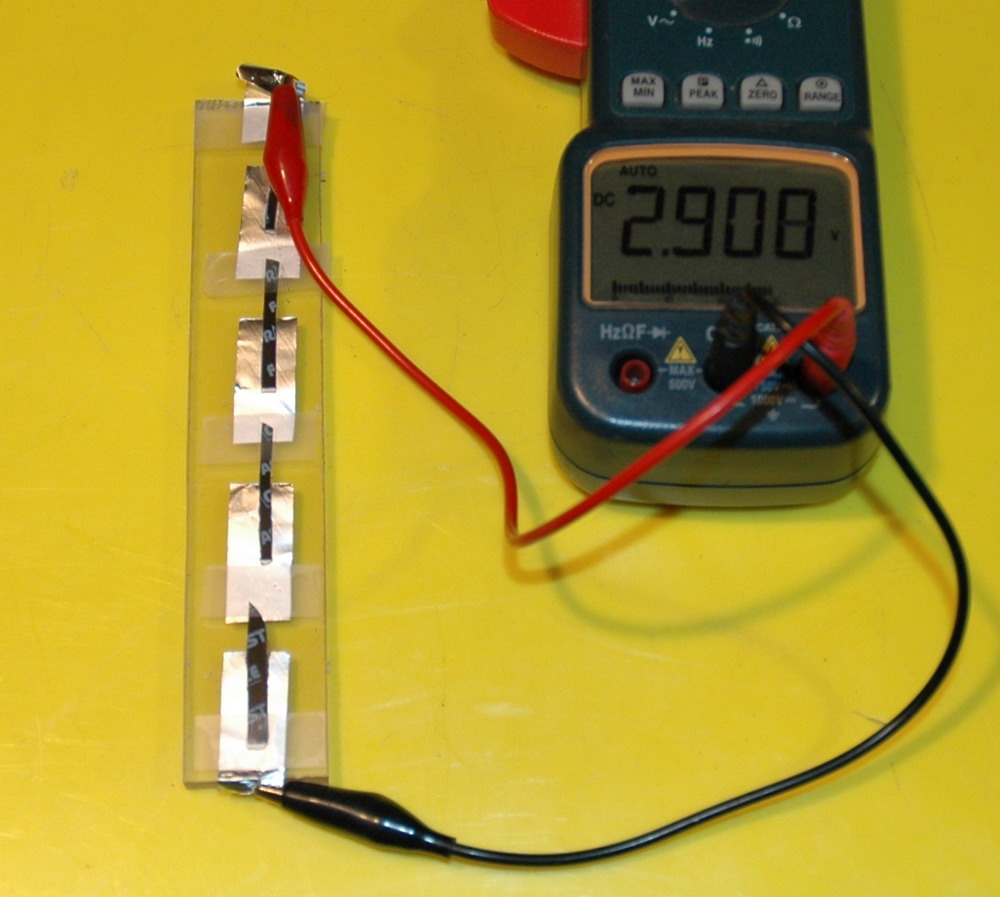
 An ECG tab and aluminium shows 0.7 V when connected to some aluminium. Current at short circuit is 1 mA. The right photo shows the result with thin strips in series generating 2.9 V. Contact between the aluminium foil and the silvered electrode is tenuous without pressure though.
An ECG tab and aluminium shows 0.7 V when connected to some aluminium. Current at short circuit is 1 mA. The right photo shows the result with thin strips in series generating 2.9 V. Contact between the aluminium foil and the silvered electrode is tenuous without pressure though.
So small batteries are possible with cheap materials. I am exploring other configurations in an effort to keep things simple and reliable. I need to think about the oxide layer forming on the aluminium as occurs in electrolytic capacitors.
 Above shows one of my batteries a year later. The aluminium foil has almost disappeared. There was little residual voltage but I could charge it up sufficiently to dimly light a LED.
Above shows one of my batteries a year later. The aluminium foil has almost disappeared. There was little residual voltage but I could charge it up sufficiently to dimly light a LED.
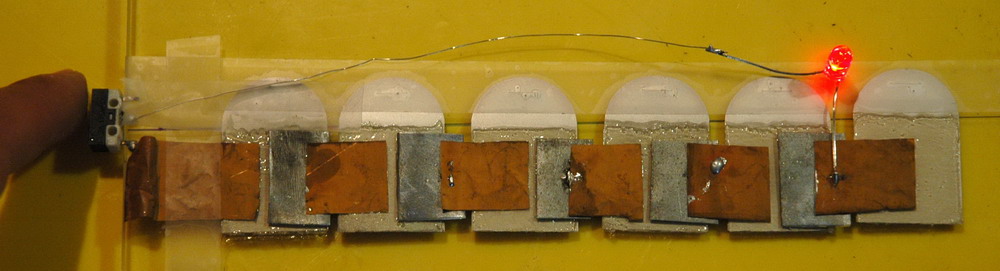 Here is another battery made from small squares of zinc plate each of which is tack soldered to copper foil squares each touching the electrolyte gel of a pad. Initial short circuit current is about 20 mA but this falls rapidly and this LED is being driven at 1.5 mA. Open circuit voltage is 4.8 V so about 0.84 V per cell.
Here is another battery made from small squares of zinc plate each of which is tack soldered to copper foil squares each touching the electrolyte gel of a pad. Initial short circuit current is about 20 mA but this falls rapidly and this LED is being driven at 1.5 mA. Open circuit voltage is 4.8 V so about 0.84 V per cell.
Related pages
Try something else
External links
Photo Date: June 10, 2006
 Electrolytic Capacitor
Electrolytic Capacitor Amanda is HOT
Amanda is HOT