Lots of fun to be had with liquid Nitrogen.
“Continue reading” for lots of pictures of liquid nitrogen fun and experiments.
A Dewar (like a big double walled thermos) containing 3 litres can be hired from a local gas supplier (livestock suppliers or BOC gases in Australia). Dewar hire was AUD$100 for a weekend and 3 litres about $12. It is not meant to be transported in an enclosed vehicle in case of accident so I use my trailer. I also have an account there for other reasons and didn’t say I wanted it for crazy experiments.
Photo above shows the Dewar with the cap. Note that this allows the gas to escape and is not a tight seal otherwise the thing will explode! The meter has a thermocouple but is way out of rated range although is reading -129 C. Liquid nitrogen (LN2) is at -196 C.
 A small balsa boat floating in the LN2.
A small balsa boat floating in the LN2.
Photos above show the classic rose-in-Liquid N2 which causes it to become so brittle and glass-like that if thrown to the ground, it will shatter. The right photo shows the brittle petals snapping off spontaneously. Lots of other things can be made had or brittle things with zany results. Eg freeze an egg, peel it and let it warm. Or, use a banana to hammer in a nail.

I’m not sure I should show this photo but here is me (carefully) throwing all the safety precautions to the wind and putting my hand in liquid N2 (for about 0.5 secs). The gas generation keeps a gas layer between you and the liquid and reduces the rate of freezing. Sensation is like a cold breeze and no discomfort but I am not pushing the boundaries here. Of course touching a solid, particularly metal, at liquid N2 temperature will give rapid severe frostbite and it might stick to you. To treat warts and other skin lesions it is applied with a cotton bud and will rapidly kill tissue.
Advice from the ‘experts’ for this somewhat risky stunt say not to get the liquid N2 in any crevices in your fingers or clothes as the liquid N2 gets forced in contact and freezing occurs. None of the family have had any frostbite incidents as we (almost always except for photos) used gloves and always used eye protection. My youngest son was using a cycling glove and got a little in though the looser weave at the back I think but no real problems. After that we stuck to the fully sealed chemical handling gloves.
Here is a banana hammer. The banana becomes extremely hard and brittle such that hammering a nail in is easy. Even worked for quite a heavy galvanised nail until the banana hammer handle broke.
I placed some liquid nitrogen in a plastic drink container and closed the lid. It took about 2 minutes for this to explode but it has taken over 5 minutes. The sharp plastic shards were only a few cm long and went a long way. It is very dangerous to approach an unexploded container. If nothing happens for over 10 minutes, throw a blanket over it might be the safest then hit it with a long stick with body/eye/ear protection. It could still have a very high pressure.
 This is what happens If you pour liquid N2 down a PVC tube to constrain it in the pool, then cover the top… It all blows out the bottom.
This is what happens If you pour liquid N2 down a PVC tube to constrain it in the pool, then cover the top… It all blows out the bottom.

A plastic cap is placed over a blob of floating liquid N2. As the gas is produced, it ‘burps’ a bubble which has fog in it. This happens repeatedly. The bubble bursts after a second or so. Kind of strange. The pool ripples are seen on the bottom indicating movement. Enough liquid N2 will cause an iceberg to form, particularly if it is constrained by a floating polystryrene ring.
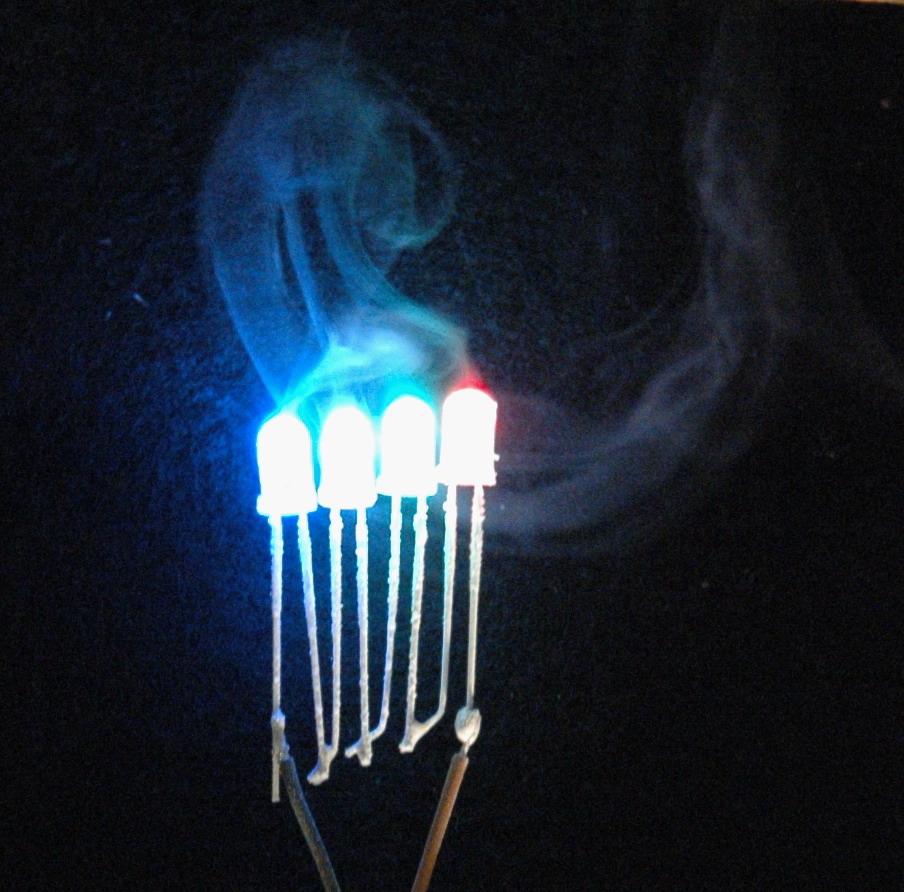
Some LED’s appearing to smoke. Actually the picture is upside down and the ‘smoke’ is just a condensation trail. You can just see the frosting on the LED’s.
A variable capacitor with air spacing after removal from the liquid N2 when water vapour frosts on it.
Here is a list of things I measured at room temp (24C) and at LN2 temp.
Resistance of copper (trigger transformer). Room 37 ohm, LN2 4.6 ohm i.e. decrease of 89 %.
Resistance of 1/2 W resistor. Room 10.02 kohm, LN2 10.70 kohm i.e. increase of 6 %
Resistance of thermistor. Room 125 kohm, LN2 >20 Mohm ie increase of 16,000 %
Inductance (RF inductor). Room 1.013 mH LN2 .810 mH i.e. decrease of 20 %
Capacitor air dielectric. Room 130 nF, LN2 171 nF. ie increase of 31 %
Most but not all of the effect is lost as soon as the LN2 is shaken out of the plates.
Things that didn’t work:
– Liquid N2 in a microwave. The microwaves are absorbed by water. (It is not true that it resonates at a particular frequency to excite water molecules – that frequency is considerably higher than 2.4 GHz). It does not seem to affect liquid N2 which should continue to boil away as normal. I also had a glass of water in there as a load.
– Model boat using N2 rocket principle. My very shoddy efforts were in vain using a syringe, tubing and needle. It is very hard to pour cold liquid N2 in to a warm place as it outgases vigorously. Also seals change size and leak or jam.
– Steam car. My model steam car didn’t generate enough power to run on sunlight alone. Replacing the water with LN2 will make it fly. The problem is the metal tank needs to be completely cooled first otherwise it bubbles too quickly and it is hard to even pour stuff in.. The pressure popoff valve may not be adequate. With some design work it shouldn’t be too hard.
– I did try to make a cloud chamber by putting an Americium source in the the neck of the N2 dewar where the cloud is normally very still. I lit the area near the emitter with a green laser but didn’t see any tracks. The heat of the source makes the cloud turbulent anyway.

This photo shows a spark UNDER LN2 with a 1 mm gap. It would spark about as readily as 20 mm in free air. The green is laser illumination for a narrow light source to go down the neck of the Dewar flask. You can see some bubbling present and ripples on the surface. HV supply was my Candy box HV.

This photo shows a 256 Hz tuning fork which is measured at 256.2 Hz at room temperature. It is made of a magnetic steel which is chrome or nickel plated and with a small NIB magnet and a pickup coil out of the turntable motor from a microwave oven, gives a nice signal for my counter for about 20 seconds.
After immersing in LN2 the frequency increases to 260.6 Hz. This is a change of 1.7% Simple coefficient of expansion of steel would account for shortening of 0.000012/K for steel. Given room temp 24 C and LN2 of -196 C this gives a 220 K range. Hence thermal expansion is .00264. This would on its own change frequency from 256 Hz to 256.67 Hz. Clearly not the full explanation. Perhaps not even any explanation as shortening will be accompanied by increased density which may compensate ? fully.
So looking at the bendy-ness of steel. I guess this is Young’s modulus and I suspect that this is the parameter that determines the vibration for a given force. The exact value is not important but the change with temperature is. This site gives the modulus for low carbon steel as being 29.5 at 70 F increasing to 31.4 at -325 F (=-198 K). This is a change of 6.4 % which would give a frequency rise to 272.4 Hz.
Since the observed frequency rise is only to 260.6 from 256.2 (1.7%) and interpolating, this suggests that the temperature of the fork at the time has risen to -34 C.
There are many assumptions, particularly about the steel type. Perhaps my logic is way off.
Nevertheless the results are plausible. Using Google to look up tuning fork and liquid nitrogen reveals many poorly documented brief physics demonstrations. The only one that gave figures was my own site here.
I have also been able to generate electricity from LN2 with a Peltier device running on LN2.
I have frozen some ferrofluid and cut it in half with a saw.
Related pages
 liquid oxygen
liquid oxygen
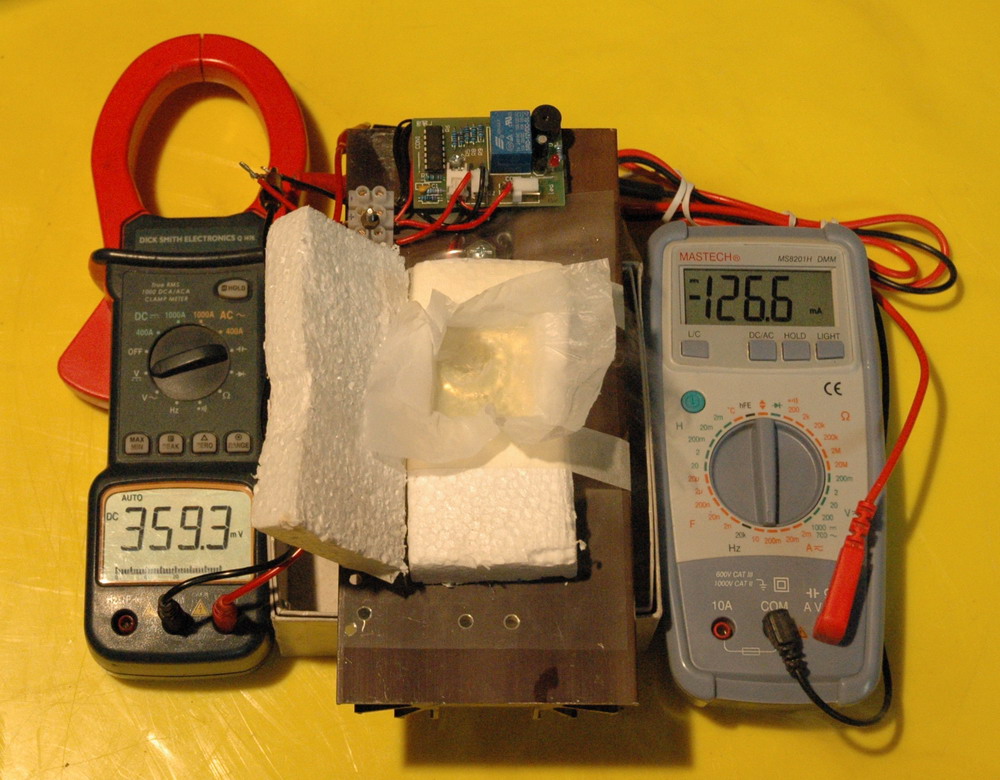 Peltier device running on LN2
Peltier device running on LN2
Try something else
External links
Liquid Nitrogen – Wikipedia
Threads and discussion about these on the 4HV forum include:
Spark in Liquid N2
Liquid Nitrogen fun
Photo Date: 2005