This 1930’s watch face had radium painted numerals to permanently glow in the dark. It has long stopped being visible as the phosphor degenerated. Strong UV light can still light it up but not as much as a bag of similar phosphor (activated zinc sulphide) shown here.
“Continue reading” for radioactive counts, more details and links…
Old watch faces had radium markings to glow in the dark called radioluminescence. These were stopped long ago due to the radiation risk to workers. The workers who used to lick the brushes to form a point and later developed cancers became an infamous example of an occupational radioactive risk.
Note the terms:
Fluorescence is the immediate emission of light less than 10 ns after the initiating exposure.
i.e. Glows while lit (most dramatically with UV)
Phosphorescence is the delayed emission of light more than 10 ns after the initiating exposure.
i.e. Glows in the dark after being lit.
Radioluminescence Glows constantly due to radioactivity. The radium watch did this when originally made.
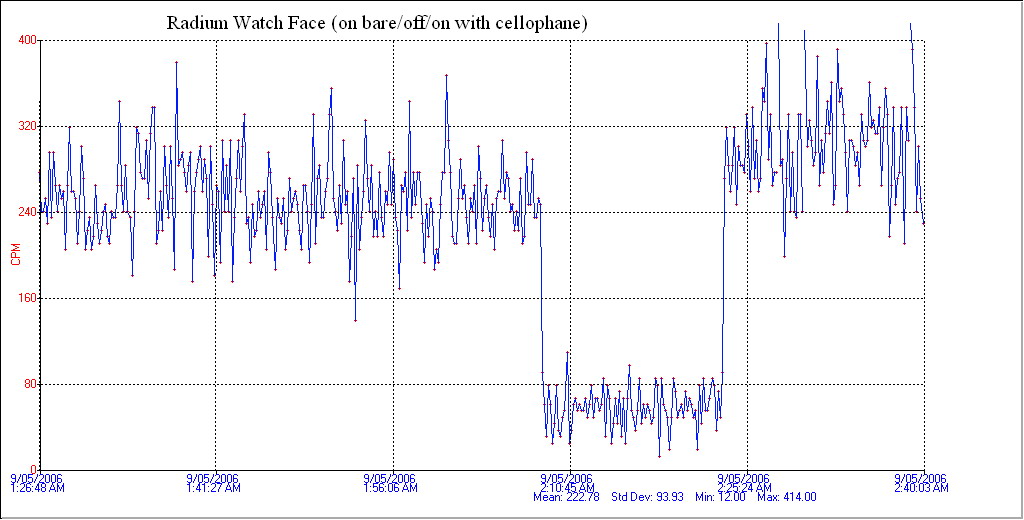
This graph shows the radiation when placed on my Geiger counter. The left side of the trace shows perhaps 260 counts per minute (CPM) with the protective cellophane off. The lower trace in the middle is background of about 60 CPM and the right trace is with cellophane back on which surprisingly reads higher. I presume that this is a positioning artefact but it may be Beta and Gamma radiation as secondary emissions from the cellophane.
Now, I recently revisited this topic. Did this really glow in the dark faintly? I had previously thought I could see the glow with dark adapted eyes but could not pick this up on a 10 minute camera exposure. The loss of glow is due to damage to the phosphor not due to loss of radioactivity from the Radium 226 which has a half life of 1600 years. So I thought that with an alpha viewing film, I might compensate for the loss of phosphor.
Above is the radium watch face behind an alpha detector film. The right photo is an 8 minute exposure clearly showing the numbers behind the film. Great! But was it the radium doing that?
Could this have been phosphorescence? This is persistent glowing of a phosphor after light exposure. To test this, I kept it in the dark for an hour to remove residual phosphorescence then ran a swipe of a violet laser across the 8 and 4 numerals. This will stimulate those numerals only to phosphorecsce.
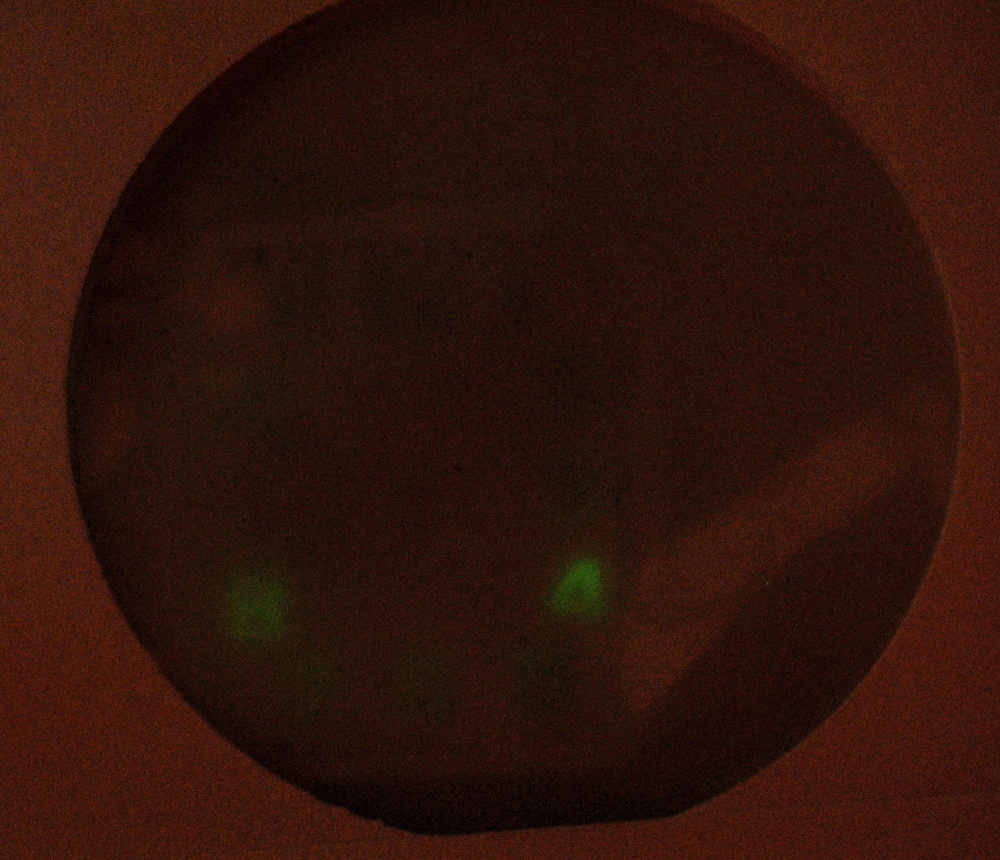 After a 10 minute exposure of the radium containing watch face behind an alpha viewing film, the only numerals that were lit were those exposed to the laser earlier. All other phosphorescence was long gone and there was no activity elsewhere.
After a 10 minute exposure of the radium containing watch face behind an alpha viewing film, the only numerals that were lit were those exposed to the laser earlier. All other phosphorescence was long gone and there was no activity elsewhere.
So this concludes that any faint glow from the watch face numerals is due to phosphorescence and not radioluminescence as it was originally made. Ohh well.
Radium is naturally occurring from Uranium (238U) breakdown. 226Ra is the most common isotope with half life 1601 years and breaks down to 222Rn. It emits Alpha radiation of 4.871 MeV.
I need to check to see if the numerals register with an americium source.
Related pages
Try something else
External links
Radium – Wikipedia
Old watch faces had radium markings
Photo Date: 2006